- Register
- Log in
-
Shopping cart
(0)
You have no items in your shopping cart.
Air-O-Cell Bioaerosol Sampling Cassette - Operating & Instruction Manual
Air-O-Cell® Sampling Cassette Operating & Instruction Manual
LA03040 Rev 4
Download as a PDF here
Buy:
Air-O-Cell® Sampling Cassette
The Air-O-Cell is a unique sampling cassette specifically designed for the rapid collection and quantitative analysis of a wide range of airborne aerosols. It collects both viable and non-viable particulate such as mold spores, pollen, insect parts, skin cell fragments, fibers (asbestos, fiberglass, cellulose, etc.) and inorganic particles. Air-O-Cell cassettes are not reusable and intended for one-time use only.
Applications
Suggested & potential applications include but are not limited to the following:
• Indoor Air Quality: Mold spores, pollen, insect parts, dust mites, skin cell fragments, plant fragments, dust, fibers, combustion emissions, etc.
• Home Inspection: Mold Contamination before or after real estate transactions.
• Flood Restoration: Evaluation of mold spore contamination before, during, and after remediation.
• Allergy Testing: Mold spores, pollen, insect parts, dust mites.
• Cleanroom Monitoring: Evaluation of low airborne dust and contaminants from personnel (skin cells, clothing fibers, cosmetics, etc.)
• Fiber Analysis: Asbestos, fiberglass, cellulose, ceramics, etc.
• Stack Emissions: Fly ash, inorganic dust, etc.
Air-O-Cell® Advantages
Provides excellent detection limits over conventional filter sampling utilizing 25 mm or 37 mm diameter filter cassettes.
• Eliminates sample loss to cassette walls known to occur with filter samples from vibration or static charge during sampling and shipment.
• Eliminates the need for direct handling or preparation of collection media or microscope slides in the field.
• Eliminates potential cross-contamination between samples and during shipping that may occur with other devices.
• Unique optically transparent and smooth collection media allows direct staining and examination by bright field, dark field, and phase contrast microscopy!!
• The sampling media is compatible with a wide range of biological stains and refractive index oils allowing for direct quantitative analysis of biological and inorganic particles.
• The Air-O-Cell will work with virtually any kind of sampling pump capable of pulling a 15 lpm (vacuum) airflow.
Principle of Operation
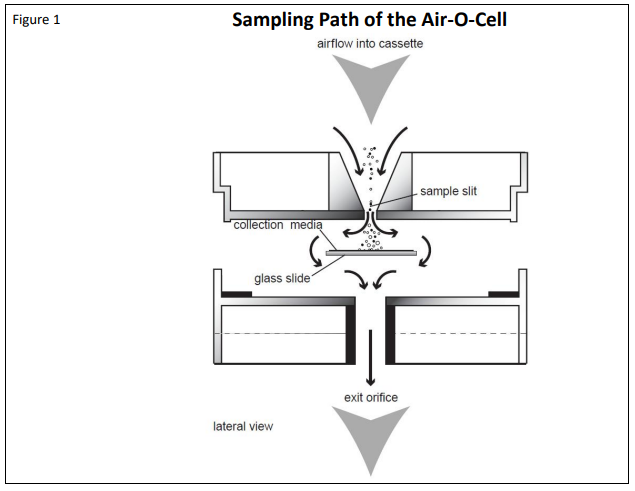
The Air-O-Cell operates on the well-established principle of inertial impaction. Particles in the air stream are accelerated as they approach the tapered inlet opening and drawn through a small slit aimed directly at a glass slide. This glass slide contains a sticky and optically clear sampling media which can permanently collect and hold particles. As the particles come through the slit, the air velocity forces the particles to impact into the sampling media, while the air stream makes a sharp 90° turn and proceeds around the slide and out of the cassette. The airflow path through the Air-O-Cell cassette is illustrated below in Figure 1.
Recommended Sampling Procedures
General
The Air-O-Cell sampling cassette is designed to operate at an optimal flow rate of 15 liters per minute. The user can employ any sampling pump capable of a minimum flow rate of 15 lpm. It is also capable of operating in any vertical or horizontal orientation, or in restricted access spaces smaller than 2 inches in diameter. As a result the Air-O-Cell is ideally suited for sampling in HVAC ducts, plenums, wall cavities, or other confined spaces.
Sampling of Ambient Static Environments
A rotameter calibrated to a primary standard, soap bubble tube/meter or a dry bubble meter should be used to calibrate the sampling pump to a flow rate of 15 lpm. Some pumps only work with specific calibration devices. Please reference the owner’s manual for your pump to verify if any special calibration methods should be employed. Because the cassette does not produce significantly measurable back pressure, the rotameter can optionally be connected directly to the pump (without the Air-O-Cell cassette in line) to calibrate the pump flow rate.
To begin sampling, remove the tape seals covering the inlet and outlet of the Air-O-Cell and place them on the side of the cassette. Then connect the Air-O-Cell cassette to the sampling pump. Turn the sampling pump on for an appropriate sample time ranging from 1 to 10 minutes and replace both seals after sampling is complete. Unlike other spore trap impaction or filter devices, the Air-O-Cell cassette can be oriented in any vertical or horizontal direction, without concern for sample loss of large particles or vibration. “Outdoor background” samples should always be collected for comparison purposes. If using an IAQ 15 Connect™ or Bio-Pump® Plus pump, please follow the owner’s manual for sampling instructions.
Sampling in HVAC Systems
A rotameter calibrated to a primary standard, soap bubble tube/meter or a dry bubble meter should be used to calibrate the sampling pump to a flow rate of 15 lpm. Some pumps only work with specific calibration devices. Please reference the owner’s manual for your pump to verify if any special calibration methods should be employed. Because the cassette does not produce significantly measurable back pressure, the rotameter can optionally be connected directly to the pump (without the Air-O-Cell cassette in line) to calibrate the pump flow rate.
To begin sampling, remove the tape seals covering the inlet and outlet of the Air-O-Cell and place them on the side of the cassette. Then connect the Air-O-Cell cassette to the sampling pump. Turn the sampling pump on for an appropriate sample time ranging from 1 to 10 minutes and replace both seals after sampling is complete. Unlike other spore trap impaction or filter devices, the Air-O-Cell cassette can be oriented in any vertical or horizontal direction, without concern for sample loss of large particles or vibration. “Outdoor background” samples should always be collected for comparison purposes. If using an IAQ 15 Connect™ or Bio-Pump® Plus pump, please follow the owner’s manual for sampling instructions.
| Table 1 Air-O-Cell Theoretical Face Velocities |
||||
| Flow Rate (lpm) | Orifice Face Velocity (fpm) | Orifice Face Velocity (mph) | Slit Face Velocity (fpm) | Slit Face Velocity (mph) |
| 15.0 | 299 | 3.4 | 3110 | 35.3 |
| 20.0 | 399 | 4.5 | 4146 | 47.2 |
| 25.0 | 499 | 5.7 | 5183 | 59.0 |
| 28.3 (1 cubic ft.) | 564 | 6.4 | 5867 | 66.8 |
| 30.0 | 598 | 6.8 | 6219 | 70.6 |
Recommended Sampling Time Intervals
Although the Air-O-Cell cassette can provide excellent detection limits over conventional filter sampling utilizing 25 mm or 37 mm diameter filter cassettes, it is also sensitive to overloading. In an appropriately loaded sample, the trace should be barely visible and transparent, but not opaque or dense. If the sample appears highly visible or opaque, additional shorter time interval samples should be collected. The recommended sampling flow rate is 15 liters per minute (lpm). As mentioned above, flow rates exceeding 20 lpm have been known to cause “bounce off” of large particles such as pollen grains. Flow rates lower than 10 lpm will not collect the small mold spores (such as Aspergillus and Penicillium) as efficiently. Recommended sampling times (at 15 lpm) for different environmental sampling conditions are given in Table 2. These are recommended and a trained on-site professional should make the final determination.
| Table 2 Recommended Sampling Intervals |
||||
| Environmental Dust Conditions | Sampling Time (minutes) at 15 lpm | |||
| Outdoor sampling on a clean windless day | 10.0 - 60.0 minutes | |||
| "Clean" office environment or outdoors (no visible dust) | 10.0 minutes | |||
| "Indoor" environment, high activity personnel | 5.0 minutes | |||
| "Indoor" environment, evidence of drywall renovation, or industrial dust | 1.0 minutes | |||
| "Indoor" environment, visible dust emissions from point sources present | 0.5 minute | |||
Recommended Laboratory Analysis Procedures
Slide Preparation
One to two (1-2) drops of staining or mounting media (lacto-phenol cotton blue is recommended for mold spore analysis) should be placed in the center of a clean pre-labeled slide. Air-O-Cell cassettes should only be opened in the laboratory. The sealing band should be cut, and the glass cover slip (containing the sample trace) removed and slowly placed on an angle with the media collection side down onto the staining solution. Do not press down on the slide during or after staining! Excess staining solution should be removed from around the edges of the cover slip with a tissue wipe or cotton swab after 10 minutes has elapsed. This will ensure even staining of the sample. It should be noted that the slide can also optionally be mounted media side up. To do this, use a drop of fingernail polish to secure the Air-O-Cell slide to the microscope slide. Then place a couple drops of stain on the media and place a cover slip on top.
About Stains
Numerous stains may be used during laboratory analysis. These include lacto-phenol cotton blue, anline blue, calbreras stain and acid fusion stain. The most common stain used for mold spore analysis is lactophenol cotton blue.
To achieve the best clarity of the sample, using stains that have little or no water content is preferred. Water can cause the sample to appear cloudy
Microscopic Examination
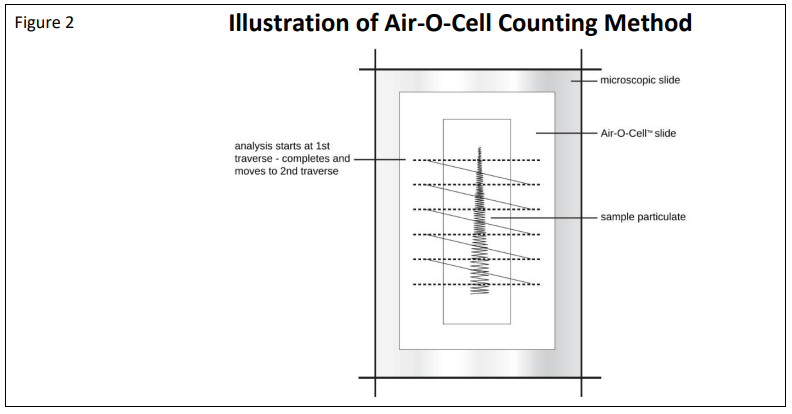
Analysis of the collected sample should be performed by an experienced Microbiologist, Aerobiologist, Mycologist or Environmental Microscopist. Counting and quantification of sample components is conducted by counting calibrated cross-sections of the deposited sample trace. The number and type of particles counted per cubic meter of air is calculated based on the length of the deposition trace, length of trace actually examined, volume of air collected, and number of particles counted.
The Air-O-Cell particle deposition area at a flow rate of 15 lpm is approximately 1.1 mm wide by 14.5 mm long yielding an approximate area of 15.95 mm2 . The width of the deposition trace will vary slightly with flow rate and media thickness, and will vary slightly in particle density from the middle to outer edges of deposition. For this reason, using the deposition trace area is not recommended for direct calculation of particle concentrations. The recommended procedure for calculating particle concentrations is based on using the Air-O-Cell trace length and microscope field diameter, and will be discussed below. One field of view counted is defined as the calibrated diameter of the microscope field of view (in mm) covering one cross-sectional pass or “traverse” across the sample deposition trace. A typical sample preparation and microscopic counting procedure is illustrated in Figure 2.
The calculation of particle concentration per cubic meter of air can be performed by using the following equations.
First, determine the actual air volume collected in cubic meters (m3) by following the calculation given in Equation 1.

Second, determine the length of sample trace counted based on the microscope field of view and number of fields of view counted. Accurately calibrate and measure the diameter of the microscope field of view using a stage micrometer slide. Remember, each microscope is different, and each different combination of ocular and objective lens must be calibrated separately. Stated lens magnifications are rarely precise. The microscopist should then record the number of complete traverses examined across the width of the deposition trace and use the formula given in Equation 2 to calculate the actual length of the deposition trace examined.

The concentrations of particles (cts/m3 ) can then be determined by using Equation 3.
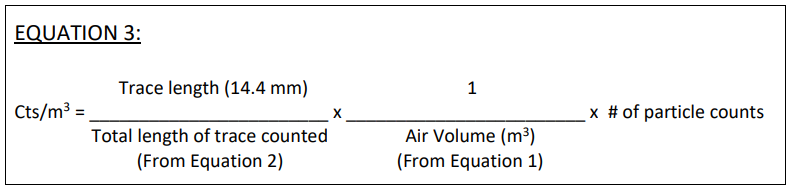
Two example calculations for mold spores and pollen grains are given below:
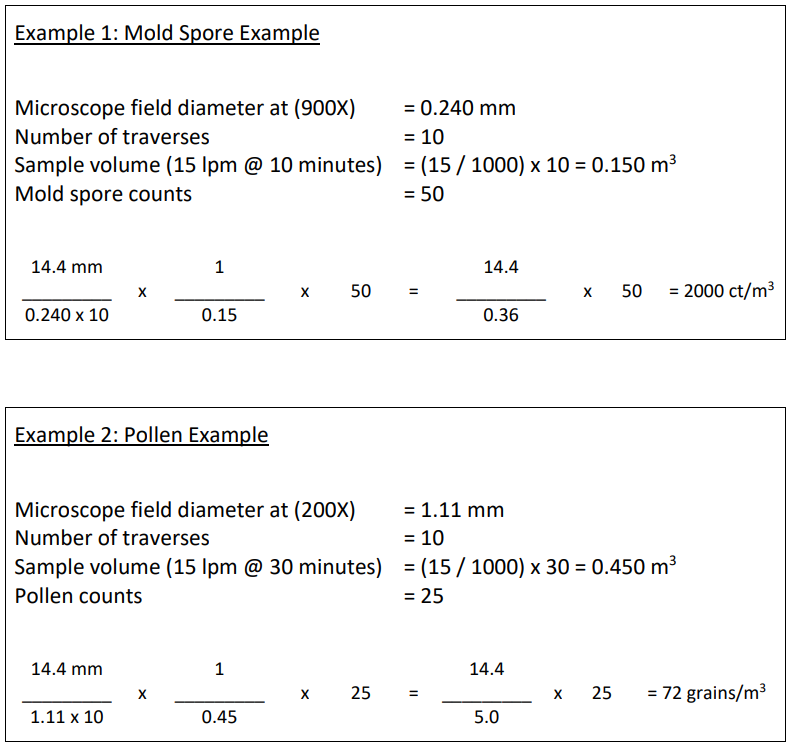
Recommended Microscopic Counting Guidelines
Counting and Identification Guidelines
• Pollen — Entire trace or 100 grains (whichever comes first) should be examined at a minimum magnification of 200X. Identification and speciation should be performed at minimum magnification of 400X.
• Mold Spores — A minimum of 15% of the entire trace should be examined or a minimum of 100 mold spores counted (whichever comes first). Identification and speciation should be performed at minimum magnification of 400X.
• Fibers — The entire trace or 100 fibers, (whichever comes first) should be examined at a minimum magnification of 200X.
• Other Aerosols — Skin cell fragments, combustion emissions, insect parts — A minimum of 10% of the entire trace should be examined or a minimum of 100 particles counted (whichever comes first).
Storage and Operating Conditions
This product should be stored at room temperature, between 60-82°F (15-28°C). Do not use product at temperatures below 32°F (0°C). If product has been exposed to freezing temperatures immediately before sampling, it is recommended to let the product acclimate to the sampling environment before use. Air-O-Cell cassettes are not reusable and intended for one-time use only
Ordering Information
| Description | Part # | |||
| Air-O-Cell Cassettes, 50/bx | AOC050 | |||
| Air-O-Cell Cassettes, 10/bx | AOC010 | |||
| Inner Wall Sampling Adapter, 10/bx | AOC-WS10 | |||
| Cassette Opener, Stainless Steel | ZA0046 | |||
| IAQ 15 Connect Sampling Pump Kit | IAQ 15 CONNECT | |||
| Bio-Pump Plus Sampling Pump Kit | ZBP-205 | |||
| TSI® 4046 Flow Calibrator Kit for IAQ 15 Connect and Bio-Pump Plus | ZBP-200-CAL | |||
| Air-O-Cell Flow Indicator Replacement for IAQ 15 Connect | 02398 | |||
| Remote Extension Tubing, 5 ft | ZBP-310 | |||
| Alcohol Wipes, 200/bx | ZA0052 | |||
Last Updated: 9/2/2022, ST